Lessons Learned About Aerosol Drug Delivery in the Era of COVID-19
COVID IN FOCUS: PERSPECTIVES ON THE LITERATURE
This CHEST series highlights specific studies in the COVID-19 literature that may warrant discourse or reading for members of the chest medicine community. Articles are written by members of CHEST Networks. You can read additional articles in this series.
NOTE: The perspectives shared in this article are those of the author(s) and not those of CHEST.
Lessons Learned About Aerosol Drug Delivery in the Era of COVID-19
By: Arzu Ari, PhD, RRT, PT, CPFT, FCCP, and J. Brady Scott, PhD, RRT, RRT-ACCS, AE-C, FCCP
Respiratory Care Network
Published: July 30, 2021
Reasonable concerns exist regarding the transmission risk of COVID-19 due to aerosol dispersion. This article provides clarity on lessons learned during the COVID-19 pandemic and summarizes strategies based on the best available evidence (Table 1).
Lesson 1: The risk of device contamination and viral transmission differs between devices.
Inhalers generate less aerosol mass due to lower emitted doses and have a short treatment time.1 The drug is also enclosed in inhalers. According to expert opinion, device contamination and viral transmission with inhalers are less than with nebulizers that require extensive cleaning after a long treatment time.1 While there is no original study comparing different nebulizers on device contamination and viral transmission, mesh nebulizers may be less prone to device contamination than jet nebulizers because they separate the drug from the patient interface, operate with electricity/battery, and have a small residual volume (Figure 1).1
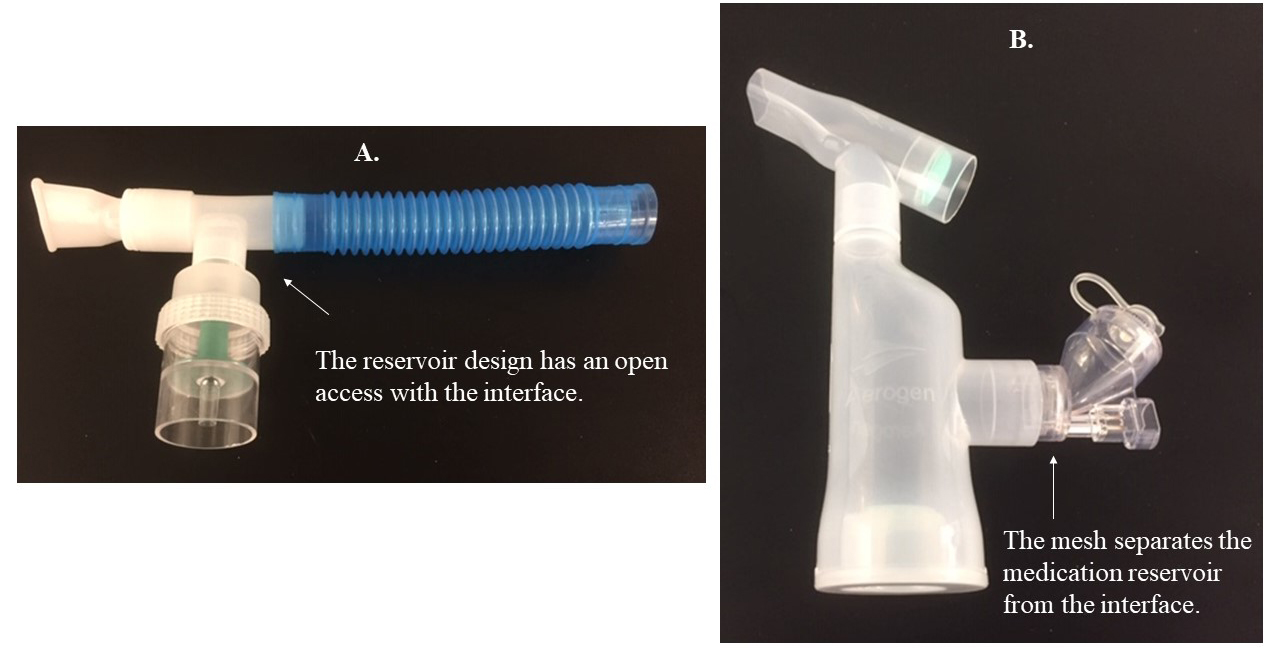 Figure 1. Nebulizers used for aerosol delivery. A) Jet nebulizer powered by a gas source. B) Mesh nebulizer powered by an electrical/battery source.
Figure 1. Nebulizers used for aerosol delivery. A) Jet nebulizer powered by a gas source. B) Mesh nebulizer powered by an electrical/battery source.
Jet nebulizers have an open medication reservoir, positioned below the gas pathway/circuit, in which infected condensate may drop into the reservoir and contaminate the jet nebulizer.1,2 The large residual volume of jet nebulizers may create a hospitable environment for pathogens when not cleaned between treatments.1,2 Also, jet nebulizers operate with an external gas flow that increases exhaled aerosol dispersion to the environment.3 Attaching a filter to the expiratory outlet of nebulizers effectively captures exhaled aerosol droplets during therapy,4,5 but evaluating the efficiency of these filters in preventing viral transmission is warranted.1,6
Lesson 2: Select an aerosol device based on the patient’s clinical status.
While inhalers are good options for spontaneously breathing patients without comorbidities, nebulizers should be used in patients who cannot perform the optimum breathing technique required by inhalers, or when the drug formulation is unavailable as an inhaler.1,6,7
In patients receiving high-flow nasal cannula (HFNC) oxygenation, or noninvasive ventilation or mechanical ventilation, clinicians should use jet nebulizers with a valved T-piece or mesh nebulizers to avoid breaking the circuit for device placement.1,6,8 Keeping the circuit intact and attaching a HEPA filter to the ventilator expiratory outlet is essential to prevent viral transmission.1,6 Since connecting pressurized metered-dose inhalers directly to the tracheotomy tube will cause cough and airway irritation, jet nebulizers with a valved T-piece or mesh nebulizers should be attached to the circuit of the high-flow oxygen device to keep the circuit intact and prevent viral transmission during aerosol therapy (Figure 2).9 If HEPA filters are used, they should be checked often and changed regularly to prevent increased resistance through the filter and increased work of breathing in patients with tracheostomy.9
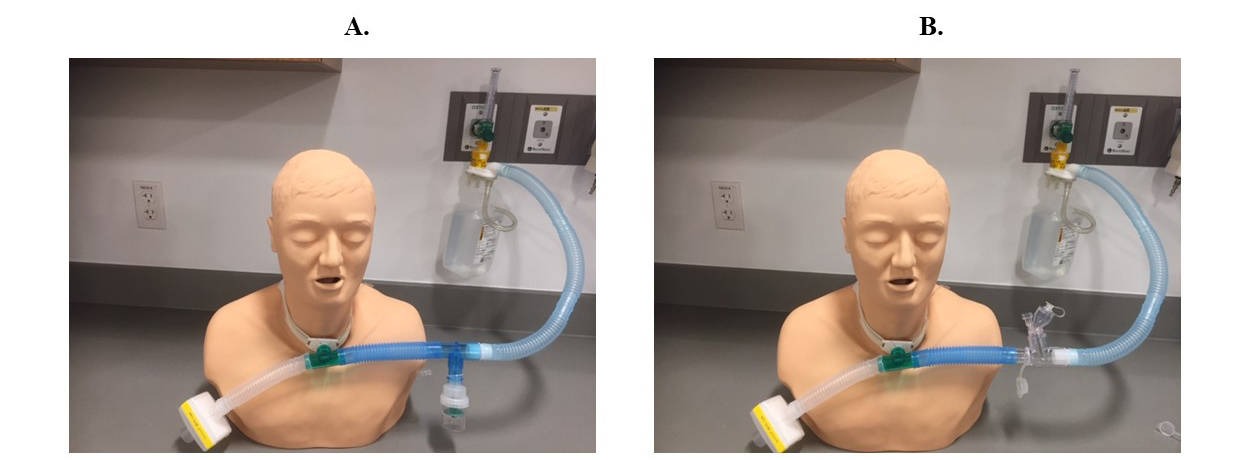 Figure 2. Nebulizer setups to reduce viral transmission during aerosol therapy in tracheotomized patients with COVID-19. A) Setup with a jet nebulizer, valved T-piece, and HEPA filter. B) Setup with a mesh nebulizer and HEPA filter.
Figure 2. Nebulizer setups to reduce viral transmission during aerosol therapy in tracheotomized patients with COVID-19. A) Setup with a jet nebulizer, valved T-piece, and HEPA filter. B) Setup with a mesh nebulizer and HEPA filter.
Lesson 3: Aerosols can be delivered through HFNC.
Concerns remain for exhaled air dispersion and viral transmission with HFNC. However, the dispersion distance of exhaled particles with HFNC is less than with Venturi and nonrebreather masks.10,11 Bacterial growth in air samples between HFNC and the simple mask does not differ significantly.12 Additionally, HFNC does not seem to increase the risk of infection via droplets.13 Due to the efficacy of HFNC in hypoxemic respiratory failure, potentially limited numbers of ventilators, and risk with early intubations, clinicians should weigh benefits against harms and consider delivering aerosolized medications through HFNC by implementing the strategies listed in Table 1.8
Lesson 4: Interface selection is as important as device selection in COVID-19.
Using face masks and tracheostomy masks increases exhaled aerosol dispersion to the environment because of open aperture and a loose fit.9,14 Instead, use a mouthpiece or T-piece for aerosol delivery to spontaneously breathing or tracheotomized patients, respectively.5,6,9
Lesson 5: Reduce exhaled aerosol dispersion to the environment through good infection control and prevention.
Using a mouthpiece, attaching filters to the expiratory outlet of nebulizers and ventilators (Figure 3) and placing a surgical mask over HFNC decreases exhaled aerosol dispersion.1,5,6,8,15-17
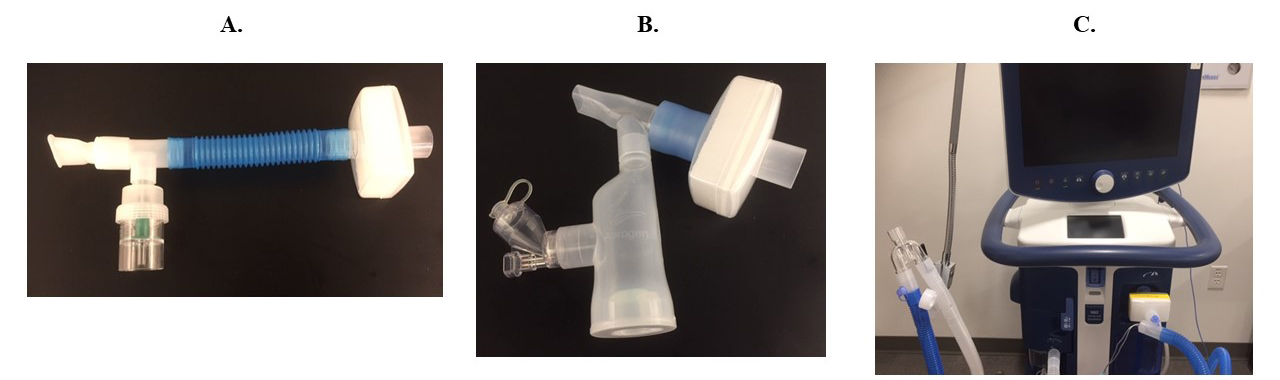 Figure 3. Filter placement to reduce aerosol dispersion. A) HEPA filter placed on the expiratory outlet of the jet nebulizer. B) HEPA filter placed on the expiratory outlet of the mesh nebulizer. C) HEPA filter placed on the expiratory limb (near the mechanical ventilator) of the ventilatory circuit.
Figure 3. Filter placement to reduce aerosol dispersion. A) HEPA filter placed on the expiratory outlet of the jet nebulizer. B) HEPA filter placed on the expiratory outlet of the mesh nebulizer. C) HEPA filter placed on the expiratory limb (near the mechanical ventilator) of the ventilatory circuit.
Patients should wear masks when possible, and use tissues during a cough or sneeze.16 Aseptic technique is vital during device preparation, cleaning, and maintenance.1 Clinicians should isolate patients in negative pressure rooms, adhere to airborne precautions, stringently use personal protective equipment, and bundle activities to minimize room entries in the era of COVID-19.1,6
Table 1. Strategies for Safe and Effective Delivery of Aerosolized Medications to Different Patient Populations
| Spontaneous Breathing |
High-Flow Nasal Cannula |
Mechanical Ventilation |
Tracheostomy |
| Avoid unnecessary aerosol drug delivery.1,6,7 |
Use HFNC for aerosol delivery before the development of severe hypoxemic respiratory failure.6 |
Do not use the pMDI to keep the ventilator circuit intact and prevent viral transmission.1,6 |
Avoid using pMDI for aerosol drug delivery to prevent viral transmission through cough and airway irritation during device placement directly to the tracheostomy tube.9 |
| Use inhalers for aerosol delivery instead of nebulizers, if the optimum technique can be performed.1,6,7 |
Use a mesh nebulizer, place it prior to the humidifier, and keep the reservoir cap closed.1,6,8 |
Use a mesh nebulizer, place it prior to the humidifier, and keep the reservoir cap closed after each use.1,6 |
Use a mesh nebulizer or a jet nebulizer with a valved T-piece. Attach them to the circuit of the high-flow oxygen delivery device (Figure 2). |
| Attach a filter to the exhalation port of the nebulizer (Figure 3) in patients who are unable to perform the optimum technique with inhalers and when drug formulation is not available as an inhaler.1,5-7 |
Make sure HFNC prongs are well-fitted and not loose.1,6,8 |
In the absence of a mesh nebulizer, use a jet nebulizer with a valved T-piece during mechanical ventilation.1,6 |
Do not use the tracheostomy mask for aerosol therapy.9 Use a T-piece and attach a HEPA filter to the expiratory outlet of the high-flow oxygen delivery system (Figure 2).9 |
| Use a mouthpiece instead of a face mask.5-7 |
Place a surgical mask on patient’s face before aerosol therapy.6,8,15 |
Attach a HEPA filter to the expiratory limb of the ventilator (Figure 3) and keep the ventilator circuit intact.1,6,17 |
Use an unassisted technique to deliver aerosols to the tracheotomized patients instead of the assisted technique unless a filter is attached to the expiratory port of the manual resuscitation bag.9 |
|
For All Patients Receiving Aerosolized Medications
Pay attention to aseptic technique during device preparation, cleaning, and maintenance. Isolate patients, administer aerosols in negative pressure rooms, and stringently use PPE during aerosol therapy.1,6,7,16
|
HFNC = High-Flow Nasal Cannula; pMDI = Pressurized Metered-Dose Inhalers; HEPA = High-Efficiency Particulate Air; PPE = Personal Protective Equipment
References
- Ari A. Promoting safe and effective use of aerosol devices in COVID-19: risks and suggestions for viral transmission. Expert Opin on Drug Deliv. 2020;17(11):1509-1513.
- Dhand R, Li J. Coughs and sneezes: their role in transmission of respiratory viral infections, including SARS-CoV-2. Am J Respir Crit Care Med. 2020;202(5):651-659.
- Hui DS, Chow BK, Chu LCY, et al. Exhaled air and aerosolized droplet dispersion during application of a jet nebulizer. Chest. 2009;135(3):648-654.
- Wittgen BPH, Kunst PWA, Perkins WR, et al. Assessing a system to capture stray aerosol during inhalation of nebulized liposomal cisplatin. J Aerosol Med. 2006;19(3):385-391.
- McGrath JA, O'Sullivan A, Bennett G, et al. Investigation of the quantity of exhaled aerosols released into the environment during nebulisation. Pharmaceutics. 2019;11(2):75.
- Ari A. Practical strategies for a safe and effective delivery of aerosolized medications to patients with COVID-19. Respir Med. 2020;167:105987.
- Ari A. Use of aerosolised medications at home for COVID-19. Lancet Respir Med. 2020;8(8):754-756.
- Ari A, Moody GB. How to deliver aerosolized medications through high flow nasal cannula safely and effectively in the era of COVID-19 and beyond: a narrative review. Can J Respir Ther. 2021;57:22-25.
- Ari A, Fink JB. Aerosol drug delivery to tracheotomized patients with COVID-19: pragmatic suggestions for clinicians. Can J Respir Ther. 2021;57:49-52.
- Hui DS, Chow BK, Lo T, et al. Exhaled air dispersion during high-flow nasal cannula therapy versus CPAP via different masks. Eur Respir J. 2019;53(4):1802339.
- Ip M, Tang JW, Hui DSC, et al. Airflow and droplet spreading around oxygen masks: a simulation model for infection control research. Am J Infect Control. 2007;35(10):684-689.
- Leung CCH, Joynt GM, Gomersall CD, et al. Comparison of high-flow nasal cannula versus oxygen face mask for environmental bacterial contamination in critically ill pneumonia patients: a randomized controlled crossover trial. J Hosp Infect. 2019;101(1):84–87.
- Kotoda M, Hishiyama S, Mitsui K, et al. Assessment of the potential for pathogen dispersal during high-flow nasal therapy. J Hosp Infect. 2020;104(4):534-537.
- Tang JW, Kalliomaki P, Varila TM, et al. Nebulisers as a potential source of airborne virus. J Infect. 2020;81(4):641-679.
- Li J, Fink JB, Elshafei AA, et al. Placing a mask on COVID-19 patients during high-flow nasal cannula therapy reduces aerosol particle dispersion. ERJ Open Res. 2021;7(1):00519-2020. Preprint. Posted online January 2021.
- Fink JB, Ehrmann S, Li J, et al. Reducing aerosol-related risk of transmission in the era of COVID-19: an interim guidance endorsed by the International Society of Aerosols in Medicine. J Aerosol Med Pulm Drug Deliv. 2020;33(6):300-304.
- Ari A, Fink JB, Pilbeam SP. Secondhand aerosol exposure during mechanical ventilation with and without expiratory filters: an in-vitro study. Ind J Resp Care. 2016;5(1):677-682.
Arzu Ari, PhD, RRT, PT, CPFT, FCCP
Dr. Ari is the Associate Dean for Research and Professor of Respiratory Care at Texas State University. Her research interest has manifested in 80 abstracts, 76 peer-reviewed articles, 16 book chapters, two clinical practice guidelines, and two books. Dr. Ari has received 29 prestigious awards for her accomplishments in research and education. She has given more than 300 presentations on aerosol drug delivery at various conferences worldwide. Dr. Ari serves on the editorial boards of the Journal of Aerosol Medicine and the Canadian Journal of Respiratory Therapy. In addition, she is the guest editor of Pharmaceutics. Dr. Ari is a fellow of the American Association for Respiratory Care and the American College of Chest Physicians.
J. Brady Scott, PhD, RRT, RRT-ACCS, AE-C, FCCP
Dr. Scott is the Director of Clinical Education and Associate Professor for Respiratory Care at Rush University in Chicago. He has been a respiratory therapist for more than 20 years, with clinical practice experience in adult emergency/critical respiratory care. In 2007, he was named the Adult Acute Care Specialty Practitioner of the Year by the American Association for Respiratory Care (AARC). Dr. Scott is a fellow of both the AARC and the American College of Chest Physicians. He has lectured at regional, state, national, and international conferences on topics pertaining to respiratory care. His research interests include simulation-based education and emergency/critical respiratory care.
ACKNOWLEDGEMENT: We would like to thank Dr. Scott Manaker for his review, revisions, and insightful suggestions on this article.
Read more COVID in Focus: Perspectives on the Literature:
Lung Transplantation for the Treatment of COVID-19 Fibrosis
Immunomodulation Therapy in Severe COVID-19 Infection: Where Do We Stand?
Clinical Outcomes of COVID-19 in Patients With COPD
Diagnosis and Containment of Patients With Suspected Lung Cancer in the COVID-19 Pandemic—What Does the Literature Say?