Ambulatory ECMO for Respiratory Support in a Patient With COVID-19 Disease
Ambulatory ECMO for Respiratory Support in a Patient With COVID-19 Disease
By: Navitha Ramesh, MD, FCCP; Devang K. Sanghavi, MD, MHA, FCCP; Si Pham, MD; Osama K. Haddad, MBBCh; and Pramod K. Guru, MD, DM
Published: May 11, 2021
Acute respiratory distress syndrome (ARDS) is a major cause of morbidity and mortality in patients with COVID-19. The management of SARS-CoV-2-associated ARDS is mainly supportive and relies upon data extrapolated from established therapies for non-COVID-19 ARDS and clinical experience from the current and previous respiratory pandemics, including venovenous extracorporeal membrane oxygenation (VV-ECMO). 1,2 Here, we describe a case of ambulatory VV-ECMO in an adult with SARS-CoV-2-induced ARDS. The patient was weaned from the ventilator while still on VV-ECMO, allowing for early mobilization and participation in physical therapy.
Case Description
A 52-year-old man with hypertension, obstructive sleep apnea, and diabetes mellitus was diagnosed with COVID-19 infection as an outpatient. His symptoms progressed, and he was admitted to the hospital 5 days after symptom onset.
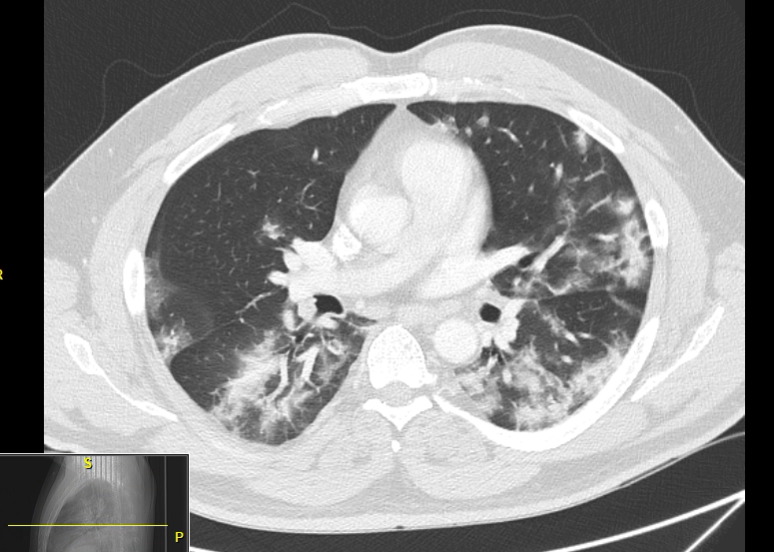 Figure 1
Figure 1Computed tomography (CT) of his chest showed extensive ground glass opacification in both lungs (see Figure 1). He was treated with supplemental oxygen via high-flow nasal cannula, remdesivir, COVID-19 convalescent plasma, and enoxaparin, with initial improvement in his symptoms. However, his condition worsened, and he was intubated on hospital day 3.
Figure 1: CT scan of the chest 4 days before the initiation of ECMO, showing bilateral extensive ground glass opacity more in the bases.
He subsequently developed extensive subcutaneous emphysema (see Figure 2) due to barotrauma and was transferred to a facility with ECMO capability. He was started on neuromuscular blockade and proned, with stable hemodynamics on inhaled nitric oxide (NO). His detailed ventilator settings are documented in Table 1.
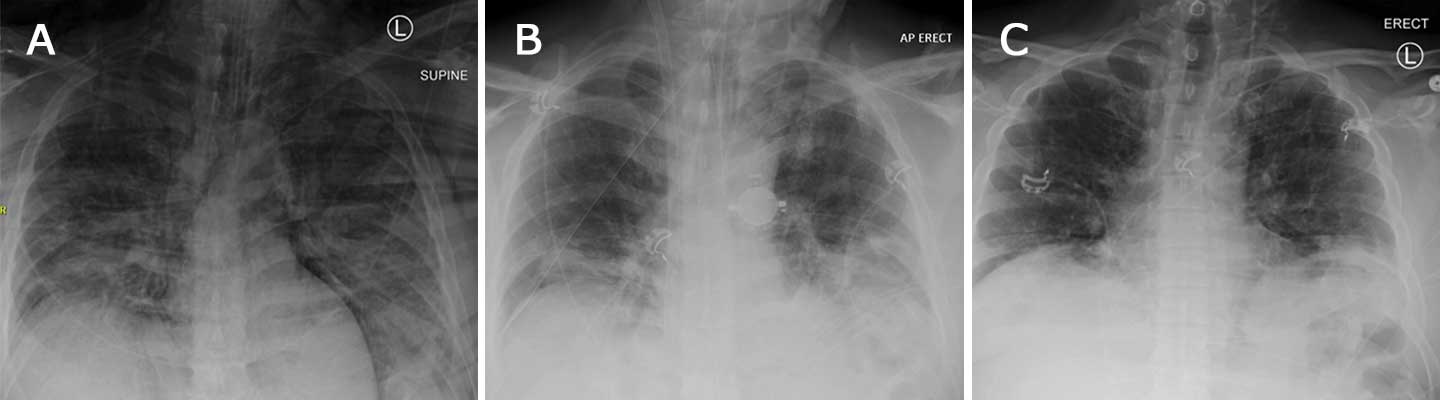
Figure 2A: X-ray of the chest on the day of ECMO initiation, showing extensive pneumomediastinum and subcutaneous emphysema. Figure 2B: X-ray of the chest on day 9 of ECMO, showing ECMO cannula in place with interval improvement of the pneumomediastinum and lung disease from COVID-19. Figure 2C: X-ray of the chest on day 11 of patient admission, when he was separated from the ECMO machine; COVID-19-related lung disease continued to improve.
Given difficulty with oxygenation and ventilation, his age, and short duration of mechanical ventilation for COVID-19 ARDS, the patient was considered for VV-ECMO. A 25 French (Fr) draining cannula was inserted through the right femoral vein and 21 Fr inflow cannula through the right internal jugular vein. ECMO flows were at 4 L/min with a sweep of 5 and FiO2 100%.
Upon ECMO initiation, the ventilator settings were reverted to ultra-lung protective P-CMV mode, FiO2 50%, PEEP 12, and PC 10. In ultra-lung protective settings, the tidal volume is further reduced from 6 mL/kg of Ideal Body Weight (as used in lung protective settings) to 2-3 mL/kg of Ideal Body Weight on the ventilator. The idea behind these settings is to give the lungs complete rest and a chance to recover while the patient is on ECMO. His medications consisted of systemic anticoagulation with bivalirudin, remdesivir and intravenous dexamethasone, empiric piperacillin/tazobactam, intravenous diuretics, and one dose of tocilizumab.
Discussion
VV-ECMO is utilized as a last resort for some patients who do not improve or deteriorate despite conventional therapies. As the COVID-19 pandemic has evolved, there has been a steady increase in ECMO use.3 Wide acceptance of utilizing ECMO for acute respiratory failure emerged during the previous H1N1 pandemic in 2009 and the publication of the CESAR trial.4 With the use of ECMO, patients experience decreased ventilator-induced lung injury and less barotrauma, allowing for alveolar healing while the ECMO machine maintains oxygenation and ventilation, leading to increased survival.2
The Extracorporeal Life Support Organization (ELSO) COVID-19 guidelines recommend against initiation of ECMO prior to maximizing traditional therapies for ARDS.3
Optimized supportive care for patients on VV-ECMO should be continued in order to achieve good patient outcomes. The ELSO guidelines recommend a lung protective ventilation strategy, targeting a plateau pressure ≤25 cmH20, RR 4-10 bpm, PEEP 10-15 cmH20, driving pressure <15 cmH20, and FiO2 <50% to maintain oxygen saturations ≥85%.5
Case Description, Continued
Over the next 12 days, the patient continued to improve, with stable ECMO flows around 4 L. He was extubated and ambulated 9 days after ECMO cannulation. He remained on VV-ECMO for an additional 3 days after extubation and was liberated from ECMO support on hospital day 12/ECMO day 12. He was eventually discharged home on hospital day 14. Table 1 describes the patient’s clinical course, ECMO parameters, and ventilator settings.
| Ventilator & VV-ECMO Settings |
Vent. Mode |
FiO2 |
PEEP |
Pressure Control |
Rate & Tidal Volume (Exp) |
Plateau Pressure |
Lung Compliance |
VV-ECMO Settings |
Hospital Day 1
Pre-ECMO |
APV-CMV |
100% |
15 |
17 |
R 20
TV 450 |
27 |
32 |
------ |
Hospital Day 1
ECMO Day 1 |
P-CMV |
50% |
12 |
10 |
R 10
TV 270 |
23 |
25 |
Flow 4 L
Sweep 5
FiO2 100 |
Hospital Day 3
ECMO Day 3 |
P-CMV |
60% |
10 |
10 |
R 10
TV 230 |
21 |
19 |
Flow 4 L
Sweep 7.5
FiO2 100 |
Hospital Day 6
ECMO Day 6 |
P-CMV |
40% |
10 |
8 |
R 10
TV 350 |
21 |
33 |
Flow 4 L
Sweep 8
FiO2 100 |
Hospital Day 9
ECMO Day 9
Pre-extubation |
P-CMV |
40% |
10 |
8 |
R 10
TV 365 |
18 |
38 |
Flow 4 L
Sweep 4.5
FiO2 60 |
Hospital Day 12
ECMO Day 12
Decannulation |
3 L NC |
------ |
------ |
------ |
------ |
------ |
------ |
Flow 3.3 L
Sweep 1.5
FiO2 30 |
Hospital Day 14
Pre-discharge |
1 L NC |
------ |
------ |
------ |
------ |
------ |
------ |
------ |
Discussion
Early mobilization is recommended when safe and feasible, in order to help improve recovery and maintain neuromuscular function.6 The unique challenges faced while attempting early ambulation of patients with COVID-19 ARDS include high disease severity with significant risk of hemodynamic instability, risk of dislodgement of catheters, increased PPE use, and potential viral transmission to health care personnel. ECMO is highly resource- and labor-intensive, in addition to being a finite resource. Hence, ongoing deliberate actions must be performed regularly in order to liberate the patients from ECMO, while also optimizing ways to liberate patients from invasive mechanical ventilation.
The disease severity, utilization of systemic corticosteroids and neuromuscular blockers, and a prolonged critical illness puts patients at an increased risk for significant neuromuscular weakness. Early initiation of the ABCDEF bundle is of utmost importance. The ABCDEF bundle is a collection of six elements that represents an evidence-based approach for clinicians to optimize patients’ recovery and ICU outcomes.7 Several guidelines recommend incorporation of the ABCDEF bundle into clinical ICU practice,7 according to the local situation and available resources.8
Case Description, Continued
The patient was subsequently followed up in Post-Intensive Care Syndrome (PICS) Clinic 1 month after his discharge. His main complaints were mild cognitive changes, in which he had difficulty remembering names and common things, along with feelings of decreased energy and endurance.
More than 50% of ICU survivors suffer from some component of PICS.9 Implementing the ABCDEF bundle to decrease delirium and mobilize the patient on ECMO early is a key step in preventing morbidity postrecovery.
Performing spontaneous awakening and breathing trials, stopping sedation, preventing delirium, and ensuring early ambulation helped our patient significantly and led to his quick discharge after decannulation from ECMO.
Summary
Early ECMO utilization in appropriate patients with COVID-19 ARDS, along with lung protective ventilation and utilization of the ABCDEF bundle, may offer a survival advantage. Early extubation before ECMO decannulation can be performed in certain circumstances, facilitating patient mobilization and participation in physical therapy under strict precautions to minimize the risk of disease transmission. Implementing the ABCDEF bundle in the care of patients with COVID-19 receiving ECMO can help reduce delirium and PICS and should be considered in all patients.
References
- Alshahrani MS, Sindi A, Alshamsi F, et al. Extracorporeal membrane oxygenation for severe Middle East respiratory syndrome coronavirus. Ann Intensive Care. 2018;8(1):3. Published January 10, 2018. doi:10.1186/s13613-017-0350-x
- Needham DM, Colantuoni E, Mendez-Tellez PA, et al. Lung protective mechanical ventilation and two year survival in patients with acute lung injury: prospective cohort study. BMJ. 2012;344:e2124. Published April 5, 2012. doi:10.1136/bmj.e2124
- Extracorporeal Life Support Organization. Accessed March 3, 2021. https://journals.lww.com/asaiojournal/Fulltext/2021/05000/Extracorporeal_Membrane_Oxygenation_for_COVID_19_.3.aspx
- Peek GJ, Clemens F, Elbourne D, et al. CESAR: conventional ventilatory support vs extracorporeal membrane oxygenation for severe adult respiratory failure. BMC Health Serv Res. 2006;6:163. Published December 23, 2006. doi:10.1186/1472-6963-6-163
- Brower RG, Matthay MA, Morris A, et al; Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301-1308.
- Abrams D, Javidfar J, Farrand E, et al. Early mobilization of patients receiving extracorporeal membrane oxygenation: a retrospective cohort study. Crit Care. 2014;18(1):R38.
- Marra A, Ely EW, Pandharipande PP, et al. The ABCDEF bundle in critical care. Crit Care Clin. 2017;33:225-243.
- Moraes FDS, Marengo LL, Silva MT, et al. ABCDE and ABCDEF care bundles: a systematic review protocol of the implementation process in intensive care units. Medicine (Baltimore). 2019;98:e14792.
- Griffiths J, Hatch RA, Bishp J, et al. An exploration of social and economic outcome and associated health-related quality of life after critical illness in general intensive care unit survivors: a 12-month follow-up study. Crit Care. 2013;17(3):R100. doi: 10.1186/cc12745
Navitha Ramesh, MD, FCCP
• Pulmonary and Critical Care Physician at UPMC Pinnacle in Harrisburg, PA
Devang K. Sanghavi, MD, MHA, FCCP
• Assistant Professor of Medicine in the Department of Critical Care at Mayo Clinic Florida in Jacksonville
Pramod K. Guru, MD, DM
• Assistant Professor of Medicine in the Department of Critical Care at Mayo Clinic Florida in Jacksonville
Si Pham, MD
• Professor and Chairman of the Division of Cardiothoracic Surgery at Mayo Clinic Florida in Jacksonville
Osama K. Haddad, MBBCh
• Fellow in the Division of Cardiothoracic Surgery at Mayo Clinic Florida in Jacksonville