Hot in Journal CHEST: February 2021
By: Divya C. Patel, DO
February 3, 2021
 Each month, we ask our Social Media Co-Editors of CHEST to weigh in on the hot topics in CHEST. This month, Dr. Patel shares her highlights of the February 2021 issue. After reviewing the issue, be sure to share your hot list on Twitter with the hashtag #journalCHEST.
Each month, we ask our Social Media Co-Editors of CHEST to weigh in on the hot topics in CHEST. This month, Dr. Patel shares her highlights of the February 2021 issue. After reviewing the issue, be sure to share your hot list on Twitter with the hashtag #journalCHEST.
I hope that you are seeing a declining number of patients with COVID-19 and that it gives you time to think more in depth about some of the other conditions that we routinely see in our practice. To that end, I am going to review three articles that may inform your practice in the coming months.
Major pulmonary complications (MPCs) are frequent after coronary artery bypass grafting (CABG) surgery. Sleep-disordered breathing (SDB) is common in patients with heart failure and coronary artery disease. In this study, the authors aimed to determine the association between SDB and MPCs by analyzing data from the prospective observational study CONSIDER AF from a single-center in Germany. Patients were tested for SDB the night before CABG using a portable device but not treated for it prior to the study. A composite end point of respiratory failure, ARDS, pneumonia, and pulmonary embolism was used to compare the SDB and non-SDB groups. A total of 250 people were included in the study and were classified by type of SDB. Previously undiagnosed SDB was diagnosed in 51% of the cohort. Sixteen percent of the patients were diagnosed with the composite end point. Patients with SDB experienced more MPCs, especially those with central sleep apnea (CSA) than those without. Multivariate analysis showed that CSA, heart failure, and history of stroke were associated with MPCs.

Distributive shock is common in the ICU and requires treatment with fluids and vasopressors. However, it can be refractory to treatment and is associated with high mortality. Angiotensin II has been studied and approved for use in high-output shock to increase mean arterial pressure (MAP) and reduce vasopressor requirements. This study aimed to assess safety and effectiveness of angiotensin II in a real-world setting by retrospectively analyzing observational data from five US medical centers. The use of angiotensin II was at the discretion of the team caring for the patients in the study. The main outcome assessment was hemodynamic response (MAP >=65 mm Hg with a stable or reduced vasopressor dose) 3 hours after angiotensin II administration. Two-hundred seventy patients received the drug during the study period primarily for septic shock. Responders and nonresponders were compared. Sixty-seven percent were categorized as responders and had lower levels of lactate and a lower rate of vasopressin use. Multivariate analysis showed that hemodynamic response to angiotensin II was associated with 30-day mortality.

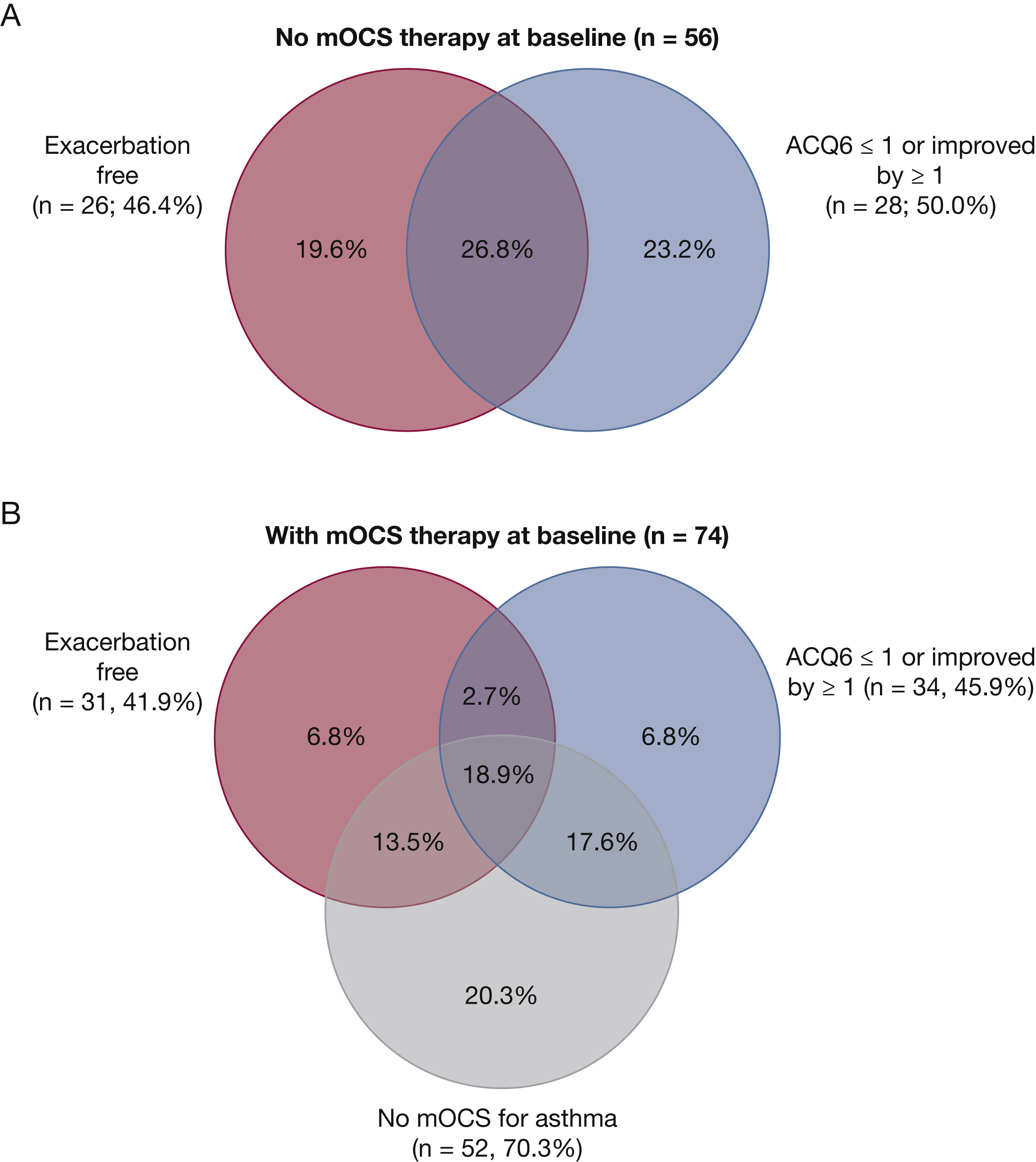
Patients with severe type 2 asthma improve only partially with high-dose inhaled corticosteroids and often take systemic steroids to control symptoms. Monoclonal antibody drugs have been used in these patients to improve symptom control and reduce exacerbation, including the monoclonal antibody drug benralizumab, which binds to an IL-5 receptor on eosinophils and basophils. Three randomized controlled trials have demonstrated its efficacy in reducing exacerbations, improving lung function, and reducing systemic corticosteroid dose in patients with severe eosinophilic asthma. In this study, the authors examined the efficacy of benralizumab for severe eosinophilic asthma in a real-world setting by retrospectively analyzing patients at one tertiary care center in the UK. Patients were classified as responders and nonresponders after 48 weeks of treatment based on 50% or greater reduction in annualized exacerbation rate, or 50% or greater reduction in daily systemic corticosteroid dose. One-hundred thirty patients were included in the study and at 48 weeks, a 73% reduction in exacerbation rate was noted. Median dose of oral corticosteroids decreased from 10 mg to 0 mg. Fifty-one patients met the definition of super responder and 112 met the definition of responder. Chronic airway infection and antidrug antibody development was noted in the 18 nonresponders.