Hot in Journal CHEST: October 2021
By: Vineesha Arelli, MD
October 26, 2021
 Each month, we ask our Social Media Co-Editors of CHEST to weigh in on the hot topics in CHEST. This month, Dr. Arelli shares her highlights of the October 2021 issue. After reviewing the issue, be sure to share your hot list on Twitter with the hashtag #journalCHEST.
Each month, we ask our Social Media Co-Editors of CHEST to weigh in on the hot topics in CHEST. This month, Dr. Arelli shares her highlights of the October 2021 issue. After reviewing the issue, be sure to share your hot list on Twitter with the hashtag #journalCHEST.
In my last blog, I had prematurely celebrated “the end of COVID.” It’s amazing what a few month’s difference makes in 2021. It has been a difficult, and somewhat surreal, 20 months with the ongoing surges. We have all been battling together and witnessing the deaths of too many people. It has been particularly frustrating to have the fight about science and the rationale for vaccinations. To all of our colleagues at the front lines, we see you and stand with you in solidarity. I hope that each and every one of you remains safe and has a chance to have some downtime. The following studies are a break from COVID-related articles.
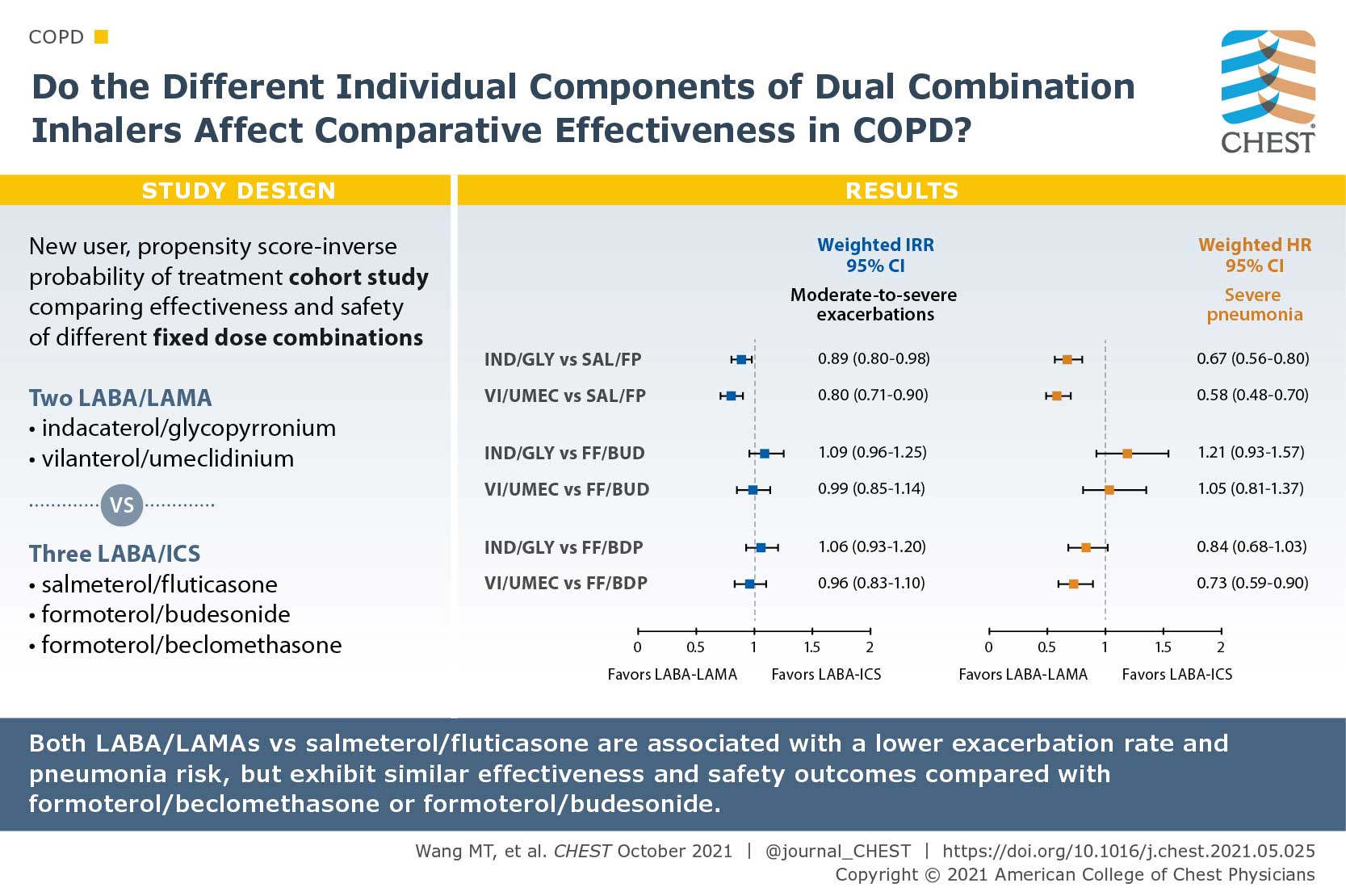
Comparative Effectiveness and Safety of Different Types of Inhaled Long-Acting β2-Agonist Plus Inhaled Corticosteroid Fixed-Dose Combinations in COPD A Propensity Score-Inverse Probability of Treatment Weighting Cohort Study
There are a multitude of fixed-dose combinations (FDCs) of inhaled long-acting β2-agonists (LABAs) plus inhaled corticosteroids (ICS) and LABAs plus long-acting muscarinic antagonists (LAMAs) for COPD. The authors questioned if the individual components of the dual combinations lead to differences of their effectiveness and safety. They used the Taiwanese nationwide health care claims from 2014 to 2017 to identify and compare the effectiveness and safety of two frequently used LABA/LAMA FDCs (indacaterol plus glycopyrronium [IND/GLY] and vilanterol plus umeclidinium [VI/UMEC]) vs three commonly prescribed LABA/ICS FDCs (salmeterol plus fluticasone propionate [SAL/FP], formoterol fumarate plus budesonide [FF/BUD], and formoterol fumarate plus beclomethasone dipropionate [FF/BDP]). Patients with COPD who used IND/GLY and VI/UMEC showed an 11% reduced annual rate of moderate to severe exacerbations compared with those who used SAL/FP, but similar rate as those who used FF/BUD or FF/BDP. Both LABA/LAMA FDCs were associated with a 27% reduced pneumonia risk. Their study suggested that comparative effects may differ by individual components of dual therapies in COPD.
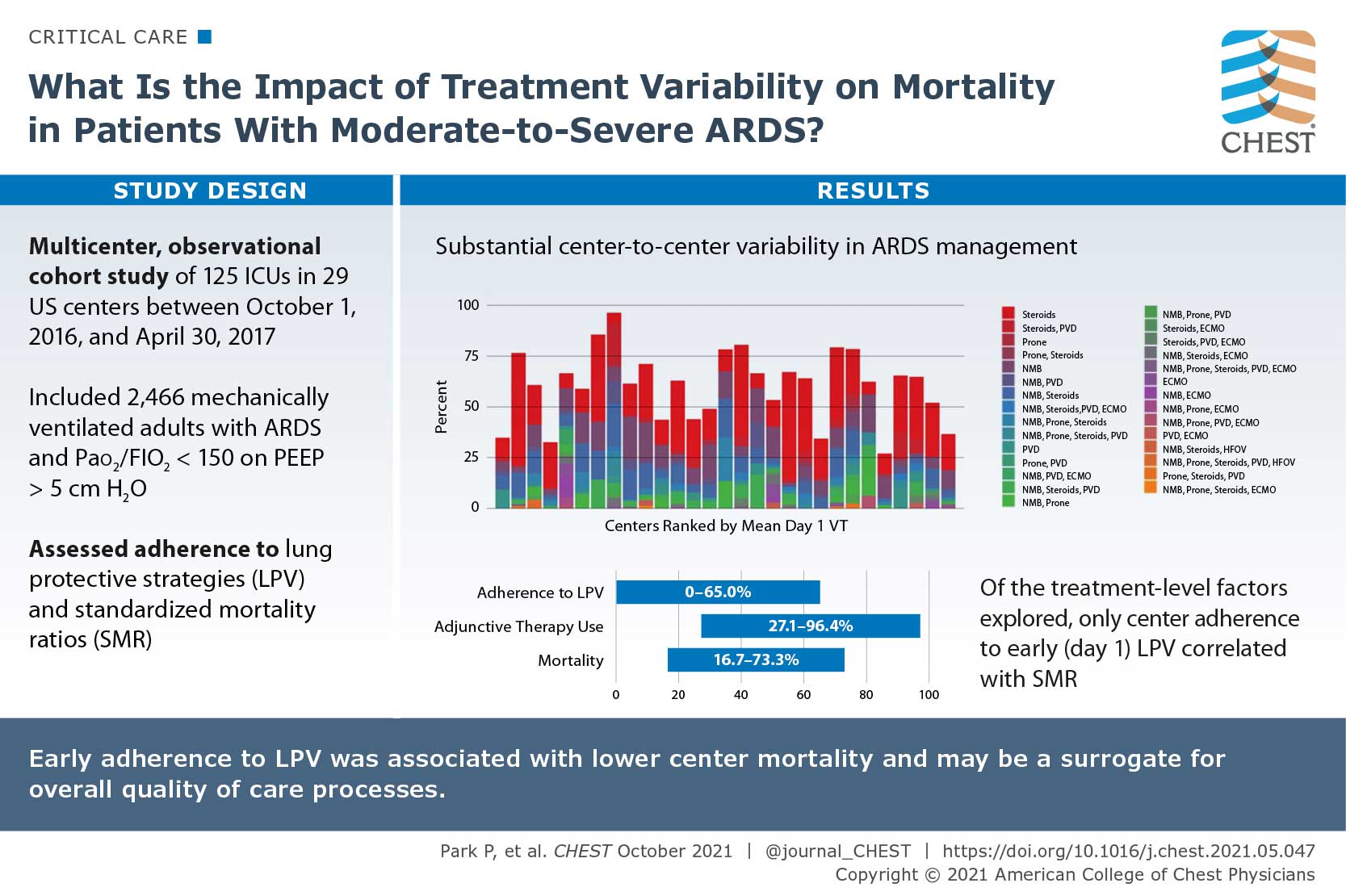
Variation in Early Management Practices in Moderate-to-Severe ARDS in the United States (The Severe ARDS: Generating Evidence Study)
There are specific interventions that show benefit in patients with ARDS, however use of the interventions are inconsistent and patient mortality remains high. The authors sought to research the impact of treatment variability on mortality in patients with moderate to severe ARDS in the United States. They conducted a multicenter, observational cohort of mechanically ventilated adults with ARDS and PaO2 to FiO2 ratio of </=150 with positive end-expiratory pressure of >/=5 cm H2O who were admitted to 29 US centers between October 1, 2016, and April 30, 2017. The primary outcome was 28-day in-hospital mortality. Out of 2,466 patients enrolled in the study, 40.7% had an in-hospital 28-day mortality. Initial adherences to lung protective ventilation was 31.4% and varied between centers (0%-65%), as did rates of adjunctive therapy use (27.1%-96.4%), methods used (neuromuscular blockade, prone positioning, systemic steroids, pulmonary vasodilators, and extracorporeal support), and mortality (16.7%-73.3%). Their study suggested that substantial center-to-center variability exists in ARDS management.
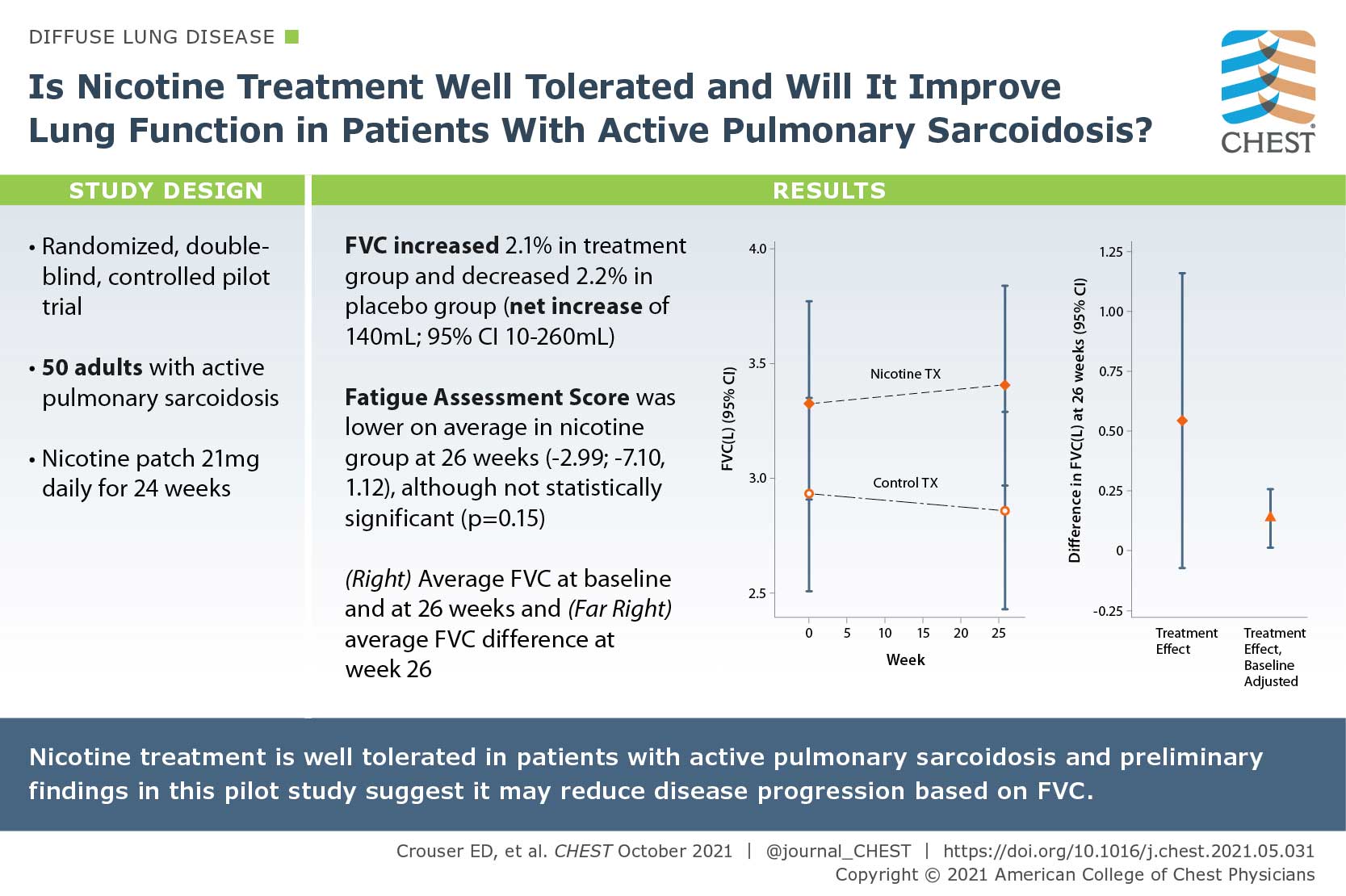
A Pilot Randomized Trial of Transdermal Nicotine for Pulmonary Sarcoidosis
Tobacco smoking has been associated with a reduced risk of developing sarcoidosis, since nicotine normalizes immune responses to environmental antigens in patients with active pulmonary sarcoidosis. In this study, the author posed if nicotine treatment is well tolerated and if it will improve lung function in patients with active pulmonary sarcoidosis. They conducted a randomized, double-blind, controlled pilot trial of daily nicotine transdermal patch treatment (21 mg daily) or placebo patch use for 24 weeks. They enrolled 50 consecutive subjects aged >/= 18 years with active pulmonary sarcoidosis based on symptoms and objective radiographic evidence of infiltrates consistent with nonfibrotic lung disease. Nicotine treatment was associated with a clinically significant, approximately 2.1% (70 mL) improvement in FVC from baseline to 26 weeks when comparing nicotine vs placebo groups at 26 weeks. The preliminary findings of this pilot study suggest that nicotine treatment may reduce disease progression based on FVC.